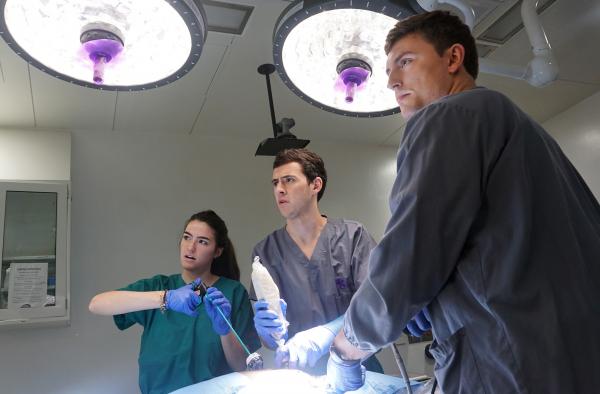
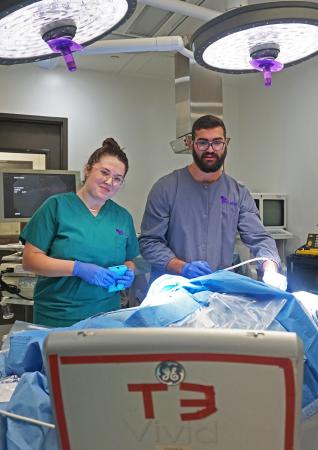
Before they won the biomedical engineering category in Monday night’s Fall 2019 Georgia Tech Capstone Design Expo, the students who comprise the team called Bullseye gave their medical device a trial run, courtesy of the Global Center for Medical Innovation (GCMI).
GCMI, a Georgia Tech affiliate, opened its T3 Labs to six teams of Capstone students, who were able to use the facilities and expertise there to evaluate their prototypes two weeks before the Expo (Dec. 2) in a real operating room setting, a critical test for young researchers designing medical devices. Bullseye was one of six teams from the Wallace H. Coulter Department of Biomedical Engineering at Tech and Emory University to test their devices at T3 Labs on GT Capstone Day at GCMI (Nov. 18).
“I was very nervous going in but I thought, ‘this is my only chance to test this!’ and just went for it,” said Bullseye member Ahmed Alnamos, whose team developed a device designed to provide a solution for patients at risk of aspiration during upper gastrointestinal endoscopy procedures (typically the first step when diagnosing esophageal and stomach diseases). The device creates a tight seal against the esophageal wall, reducing aspiration by preventing the backflow of stomach contents further up into the esophagus and eventually into the trachea.
“There are more than 50-million of these procedures annually in the United States and aspiration, although the risk is less than one percent, is devastating for the lungs when it does occur because they are not meant to withstand the acidity of the gastric contents,” explained Alnamos. “When that volume reaches the lungs, it can cause aspiration pneumonia and possibly death.”
Like any good dress rehearsal, the experience at T3 Labs proved invaluable and helped all the teams prepare for their big night.
“Our device is intended for one-time use,” said Bullseye’s Nishani Kanthasamy, who added that she and her teammates were able to perfect their prototype in the time they had before the Expo.
That’s exactly that T3 was developed for – the full-name of the facility is Translational Testing and Training Labs. It’s a place where physicians go to learn how to use new cutting edge devices and equipment.
“About 80 percent of our work here is preclinical research,” said Evan Goldberg, program director for GCMI. “From proof of concept all the way to safety and efficacy studies. We enjoy supporting these Capstone students, who have a great opportunity to test their devices in practical, clinical situations.”
A team called Leading Lap set out to reduce complications associated with initial trocar placement during laparoscopic procedures, and while their device didn’t work as planned, the team got a lot out of the experience.
“It’s one thing to look at numbers in a research paper and another thing altogether to use it, hands on in real time – you get a new appreciation and better understanding of what needs to be done going forward,” said Leading Lap team member Will Kannick.
Similar sentiments were echoed by the other BME Capstone teams at T3 Labs, such as DISCmissed, a group of four students that created a device for enhanced vertebral disc removal.
“There are roughly 350,000 of these procedures done a year in the United States and according to the surgeons that we’ve spoken with, disc removal is the most taxing portion of the spinal fusion procedure,” explained Gwyn Lando, whose team realized early on that testing their prototype would be a challenge. “We heard from some surgeons that the resected material is almost spongy but it pulls apart like crab mean. We were going to try to pull crab meat from between two 3D-printed vertebrae.”
Consequently, she said, “Being able to test the device in a cadaveric setting thanks to GCMI was the best opportunity we could have been afforded.”
Media Contact
Jerry Grillo
Communications Officer II
Parker H. Petit Institute for
Bioengineering and Bioscience
Latest BME News
Jo honored for his impact on science and mentorship
The department rises to the top in biomedical engineering programs for undergraduate education.
Commercialization program in Coulter BME announces project teams who will receive support to get their research to market.
Courses in the Wallace H. Coulter Department of Biomedical Engineering are being reformatted to incorporate AI and machine learning so students are prepared for a data-driven biotech sector.
Influenced by her mother's journey in engineering, Sriya Surapaneni hopes to inspire other young women in the field.
Coulter BME Professor Earns Tenure, Eyes Future of Innovation in Health and Medicine
The grant will fund the development of cutting-edge technology that could detect colorectal cancer through a simple breath test
The surgical support device landed Coulter BME its 4th consecutive win for the College of Engineering competition.